The immunogen against hepatitis B has been used in South Korea, New Zealand, Singapore, Australia, Philippines, Taipei of China, Thailand and Hong Kong, with positive impact on patients, thus receiving the endorsement of their respective regulatory authorities.
In 80 percent of the people, the presence of the virus in the blood had decreased considerably, to less than 10,000 particles per milliliter.
This means that, although the patient is still infected, the risk of suffering complications such as fibrosis, the end result of which can be cirrhosis and liver cancer, is reduced.
Even five years after administering the vaccine, 80 percent of the individuals keep the viral load under control, and in 50 percent the viral load is undetectable.
HeberNasvac -which has the collaboration of the French company Abivax- received recognition for its effectiveness in hepatitis B therapy at the Annual Congress of the American Society of Liver Diseases.
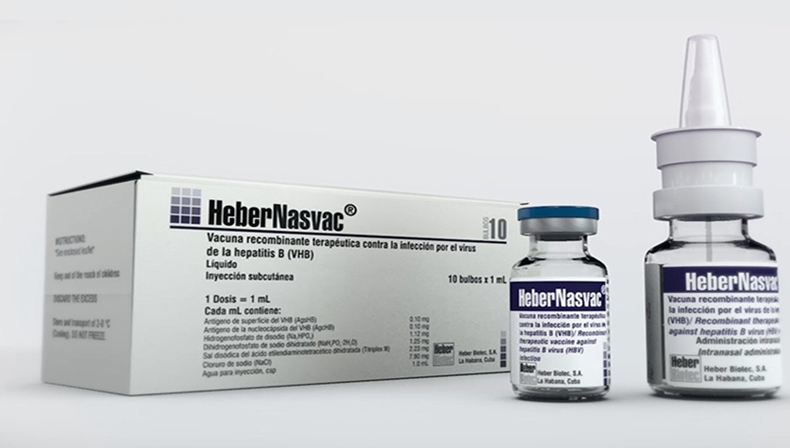
This immunogen, whose scheme in Cuba consists of nasal administration, combined with the subcutaneous use, is not only effective but also highly safe, with very few adverse reactions, and an administration period of five months, much shorter than that of interferon, administered for one year, and antivirals, which are necessary for life.
The decrease in the incidence of hepatitis B in Cuba means that it is not considered a health problem, and that Cuba aspires to its total elimination by 2030, in line with the aspiration of the World Health Organization.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION




Escambray reserves the right to publish comments.