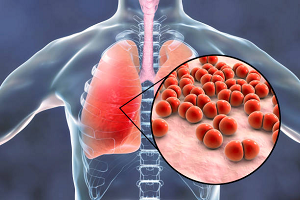
According to the analysis, there are currently 61 candidate vaccines in various phases of clinical study to deal with diseases caused by pathogens within the list of bacteria characterized as priorities by the entity.
In this way, the Antillean product is inserted in a list published by the United Nations agency along with other preparations in clinical trials designed to combat pneumococcus in countries such as the United States, the United Kingdom, Switzerland, Austria, China and Brazil.
A few months ago, the general director of the Finlay Institute of Vaccines, Doctor Vicente Vérez, declared that the Cuban produced vaccine is very close to being authorized to be applied to children.
As part of an intervention study, he stated, it was applied to children in the province of Cienfuegos (230 kilometers southeast of this capital), after which there was a significant reduction in respiratory infections in that territory.
According to statements made to Prensa Latina by Daniel García, director of the Chemical and Biomolecular Synthesis Laboratory of the Faculty of Chemistry of the University of Havana, production of Quimi-Vio is currently being resumed and its generalized clinical evaluation will soon begin.
Actually, he said, it’s many vaccines in one, as it fights all seven strains of the Streptococcus Pneumonia bacteria, which causes pneumonia, meningitis and sepsis in children.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION




Escambray reserves the right to publish comments.