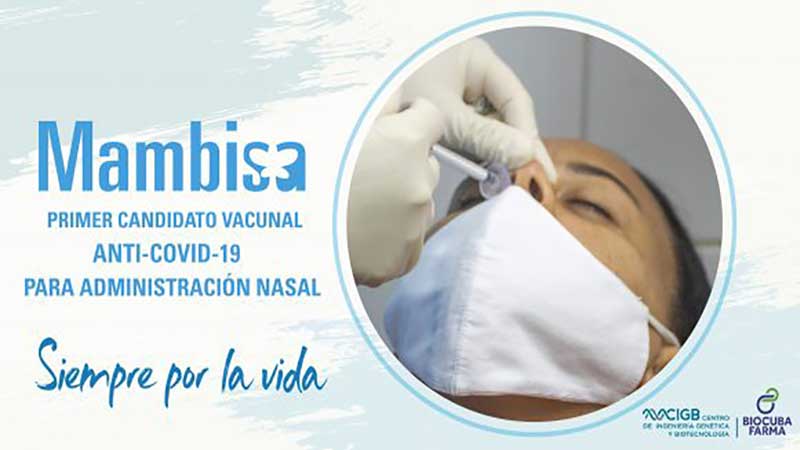
The study included 1 thousand 41 volunteers, out of whom over 70 percent saw their inhibitory capacity and antibody titles increase four times, which surpasses initial expectations.
Half the volunteers received an Abdala vaccine shot via intramuscular, and the other half were vaccinated with Mambisa, with the latter ones proving the effect of the candidate vaccine.
This evidence allows for the preparation of a final report on the clinical research and the request to the local regulatory authority to authorize the emergency use of the vaccine.
The Cuban homegrown vaccine has been considered to be administered in convalescents and as a booster. The vaccine stimulates local immunity of nasal mucosa, the main entry of the coronavirus.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION




Escambray reserves the right to publish comments.