Formal contacts with WHO on this immunogen, developed by the Center for Genetic Engineering and Biotechnology (CIGB), began in March.
The Biotechnological and Pharmaceutical Industries Group (BioCubaFarma) reported on its Twitter account that the UN agency has already been informed about the completed documentation to be reviewed by experts.
In mid-February this year, BioCubaFarma President Eduardo Martinez explained that the company was working on the material, made up of several chapters with results on clinical and preclinical research, the pharmaceutical development and everything associated with the production facilities, an aspect to which adaptations were made.
In operation since November 2021, the CIGB-Mariel Biotechnological Industrial Complex is considered the most modern facility of its kind in Cuba and one of the most advanced industries in Latin America and the Caribbean.
The objective is that WHO representatives will also visit the production lines of that complex where the necessary inspection will be carried out to later obtain the permit and its inclusion in the list of products recognized by the health organization, Martinez stressed.
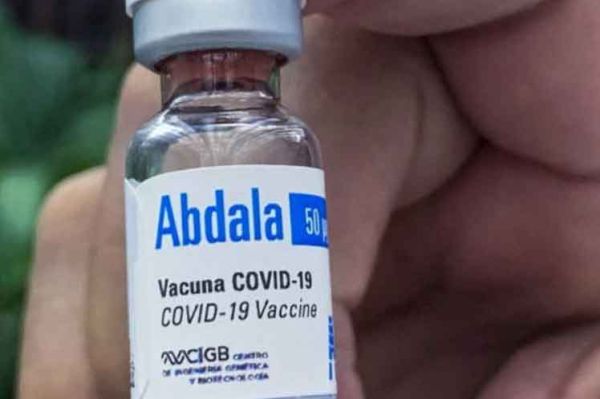
Cuba has administered 35 447 065 doses of its homegrown Soberana 02, Soberana Plus, both produced by the Finlay Vaccine Institute, and Abdala (CIGB-66) vaccines, according to data from the Ministry of Public Health.
Until April 2, 10 637 288 people had received at least one shot of any of these vaccines, included Covid-19 convalescents to whom a single injection of Soberana Plus was administered.
A total of 9 917 183 Cubans have been fully vaccinated, which account for 89.5 percent of the country’s population (approximately 11 300 000). The Covid-19 booster dose has reached 6 339 254 nationals.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION




Escambray reserves the right to publish comments.