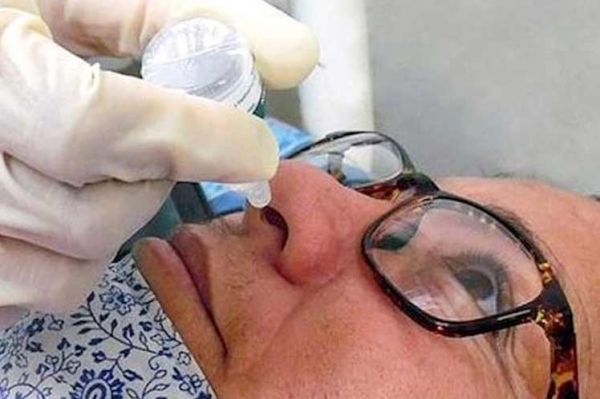
Cuba’s nasal vaccine candidate against COVID-19, Mambisa, is today on the list of five such products created in the world and is considered safe.
Marta Ayala, director of the Genetic Engineering and Biotechnology Center(CIGB), explained that Mambisa is the only one in that list that is based on the technological platform of recombinantly produced antigens.
Among its main advantages are safety, because the adverse effects registered are slight and multiple doses of it can be administered in order to reinforce the immune response over time, the expert pointed out.
Another benefit, said Ayala, lies in its potential to induce this type of action in the nasopharyngeal mucosa, something particularly convenient, since COVID19 is a disease whose entry point is through the respiratory tract.
One of the proteins that are part of the aforementioned product, the nucleocepsid or protein, makes up the nucleus of the virus, which has the ability to stimulate the immune response when combined with the RBD protein, noted the CIGB director, who was quoted by Granma newspaper.
In this sense, the director of Biomedical Research of the CIGB, Gerardo Guillen, explained that Mambisa completed the phase I of clinical trials in 88 volunteers at the National Toxicology Center.
The vaccine showed a good level of safety and the preliminary results also revealed an effective immunological action, Guillen explained.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION





Escambray reserves the right to publish comments.