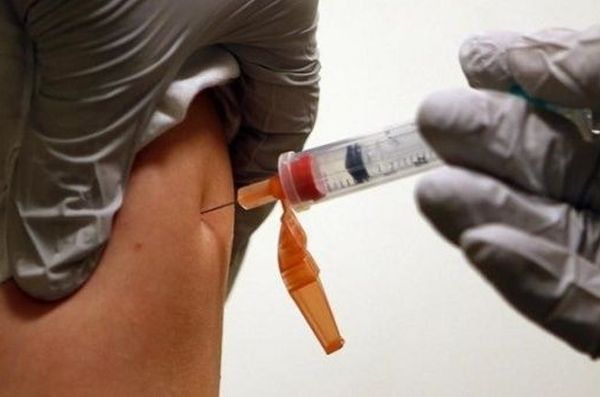
The drug is injected weekly and has been shown to have fewer side effects than many other HIV treatment methods
The world’s first long-acting fusion inhibitor treatment for HIV has been developed and approved for use in China, after years of studies and trials.
The China Food and Drug Administration approved the domestically developed drug Albuvirtide for use in HIV treatment in the country.
The drug is injected weekly and has been shown in trials to be safer and have fewer side effects than many other HIV treatment methods. It is a fusion inhibitor that can be used alongside antiretroviral drugs. It will also make HIV treatment significantly cheaper.
Albuvirtide, developed by Frontier Biotechnologies, works by binding to the HIV gp41 envelope protein. It is closely related to an older treatment called Enfuvirtide, which is now rarely used as it relies on a series of multiple daily injections.
China’s first domestically developed drug offers new HIV patients a new treatment option. We hope to dispel the fact that China has not developed good anti-AIDS medicine,” Xie Dong, the chief scientist of Frontier Biotechnologies said according to Science and Technology Daily.
According to Xinhua, 718,270 people currently suffer from HIV in China.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION





Escambray reserves the right to publish comments.