The Twitter account of the scientific center thanked those who volunteered for the trials for their trust in the process, which took place at the Saturnino Lora Hospital, in eastern Santiago de Cuba.
Nearly 700 volunteers from work centers and schools in Santiago submitted themselves to the study, which revealed a positive immunological response.
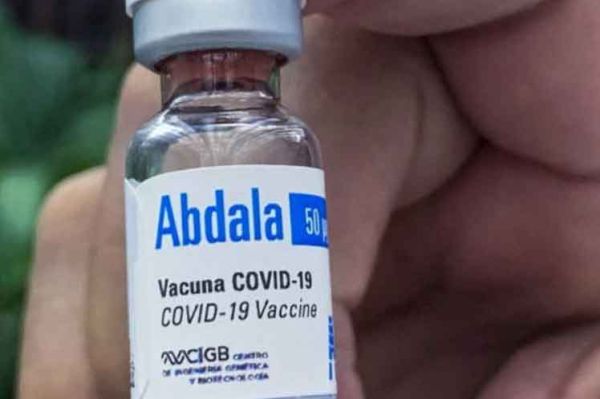
In phase I of clinical trials the candidate vaccine showed no adverse reactions and appropriate safety in 132 volunteers, who only referred to a slight pain in the intramuscular injection zone.
After a second phase concluded, a third stage proved an efficacy level of 92,28 percent in preventing the symptomatic disease.
The administration of the Abdala vaccine in adults was authorized in July 9, 2021 and in children between 2 and 11 years of age on October 27 that year.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION




Escambray reserves the right to publish comments.