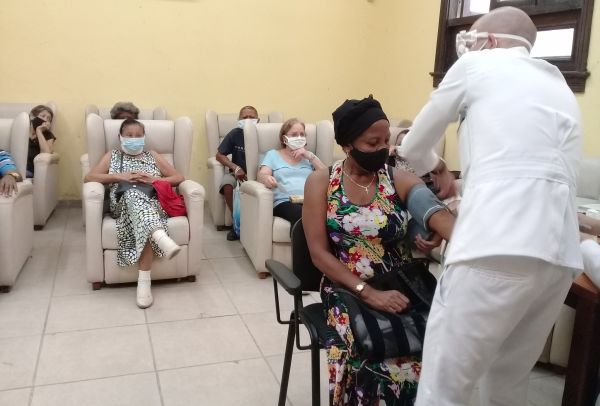
The phase III clinical trial of the Cuban COVID-19 candidate Soberana 02 continues this week in Havana.
The Biotechnology and Pharmaceutical Industries of Cuba Business Group (BioCubafarma) reported on Twitter that some 4,500 people of the 40,000 expected to participate at this stage had been vaccinated on the first week.
“Only some mild adverse effects and general discomfort are reported,” the group reported.
40 vaccination centers participated in the trial last week, a figure that should increase in the coming days in the municipalities of the Cuban capital selected.
In the first week of “piloting and adjusting”, the established protocol and good clinical practices were strictly complied with, indicated the information, cited by the Agencia Cubana de Noticias news agency.
Soberana 02 began its clinical trials in October 2020. Two months later it advanced to the second phase of the study. After obtaining and demonstrating good immunogenicity results, it received the green light to begin the definitive and final clinical trial.
Doses of the drug are currently being administered to volunteers between 19 and 80 years of age to identify the presence of neutralizing antibodies to the virus that causes COVID-19.
Cuba intends to immunize its entire population against the coronavirus this year. Its biopharmaceutical industry is developing five vaccine candidates: Soberana 01, Soberana 02, Abdala, Mambisa, and Soberana Plus, the latter recently presented and intended for convalescents from COVID-19 and as booster doses to other vaccines.
 Escambray ENGLISH EDITION
Escambray ENGLISH EDITION





Escambray reserves the right to publish comments.